How this study works
Step 1: Sign Up
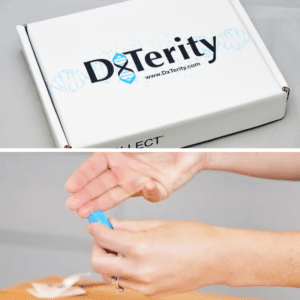
Step 2: Complete a Kit
Complete a fingerstick kit from the comfort and convenience of home and drop it in the mail.
Step 3: Share Your Experience
Let us know how you are feeling, and complete a short survey online.
